
Chemistry, 18.09.2021 01:00, annemcnair217
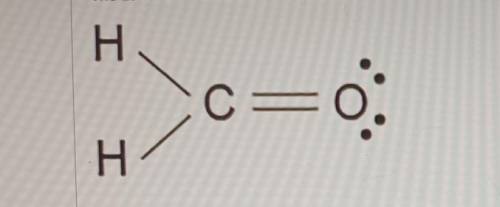
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 points)
O Oxygen is the least electronegative of the three atoms.
O Carbon has a total of four bonded pairs of electrons around it.
O Oxygen has four pairs of non-bonding innermost shell electrons.
O Carbon has an incomplete octet as it transfers an electron to each hydrogen.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, ashleylit8
4moles of nitrogen gas are confined to a 6.0 l vessel at 177 °c and 12.0 atm. if the vessel is allowed to expand isothermally to 36.0 l, what would be the final pressure?
Answers: 3


Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
Do you know the correct answer?
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 p...
Questions in other subjects:


Mathematics, 31.10.2019 07:31

Health, 31.10.2019 07:31

Physics, 31.10.2019 07:31

History, 31.10.2019 07:31










