
Chemistry, 17.09.2021 19:30, Student3220
In an experiment, a student measured the maximum mass of sugar in grams that can dissolve in 100 mL of water at five different temperatures. (You will answer the questions below using this experiment and graph. Hint: Use Units) I'm not really understanding, so any type of tips would really help. Thank you!
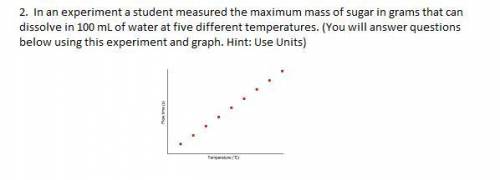
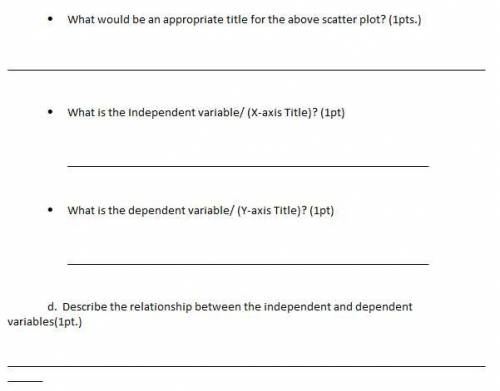

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
In an experiment, a student measured the maximum mass of sugar in grams that can dissolve in 100 mL...
Questions in other subjects:


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01










