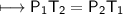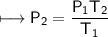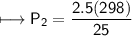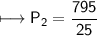Chemistry, 17.09.2021 08:30, aischa282005
A spray paint can with a pressure of 2.50 atm is trapped inside a burning
factory. Its initial temperature is 25°C. What is the final pressure of the can
if the fire reaches a temperature of 635°C? Assume that the volume of the
can remains constant!

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 13:50, isaac7454
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1
Do you know the correct answer?
A spray paint can with a pressure of 2.50 atm is trapped inside a burning
factory. Its initial tem...
Questions in other subjects:




Chemistry, 19.05.2020 22:10



Mathematics, 19.05.2020 22:10


Mathematics, 19.05.2020 22:10