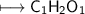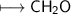Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
Do you know the correct answer?
An unknown organic compound composed of carbon, hydrogen and oxygen was analyzed and found to be 50....
Questions in other subjects:



Biology, 16.07.2019 07:50


English, 16.07.2019 07:50

Mathematics, 16.07.2019 07:50



English, 16.07.2019 07:50

Health, 16.07.2019 07:50