
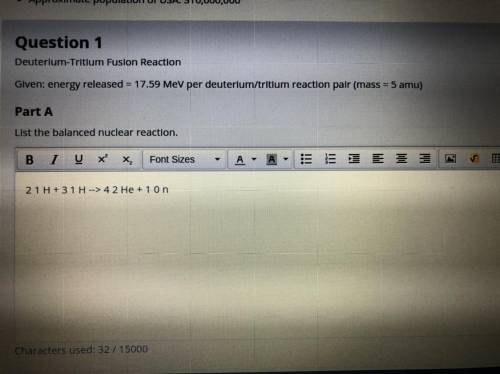
Part A. Deuterium-tritium fusion reaction. Given: energy released = 17.59 MeV per deuterium/tritium reaction pair (mass = 5 amu)
Part B. Determine the energy released per kilogram of fuel used. Given MeV per reaction, calculate energy in joules per kilogram of reactants. Consider 1 mole of tritium plus 1 mole of deuterium yo be a mole of “reactions” (total molar mass = 5 grams)
Part C. Determine the mass of fuel required for the expected energy consumption in the United States for the next 10 years. Energy used per person per year in the United States = 3.5 x 10^11 joules. Base your calculations on a current population of 310,000,000

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3


Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Do you know the correct answer?
Part A. Deuterium-tritium fusion reaction. Given: energy released = 17.59 MeV per deuterium/tritium...
Questions in other subjects:

Mathematics, 01.09.2021 03:10

History, 01.09.2021 03:10


Mathematics, 01.09.2021 03:10

Mathematics, 01.09.2021 03:10


Mathematics, 01.09.2021 03:10

Business, 01.09.2021 03:10


Mathematics, 01.09.2021 03:10






