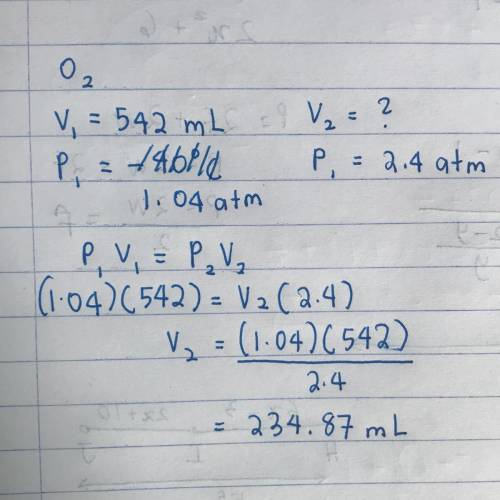When 542 mL of O2 gas at 30°C and 1.04 atm
is cooled to –40 °C and the pressure is in-
creas...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1


Do you know the correct answer?
Questions in other subjects:


History, 10.09.2019 21:30

Biology, 10.09.2019 21:30

History, 10.09.2019 21:30


Chemistry, 10.09.2019 21:30

Mathematics, 10.09.2019 21:30

Mathematics, 10.09.2019 21:30