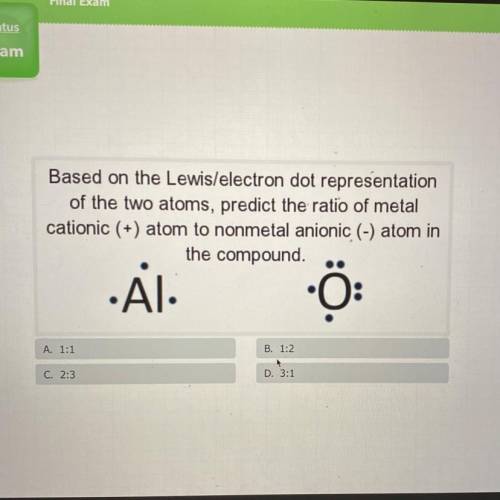
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Social Studies, 16.01.2020 17:31




History, 16.01.2020 17:31

Mathematics, 16.01.2020 17:31

Mathematics, 16.01.2020 17:31



Mathematics, 16.01.2020 17:31