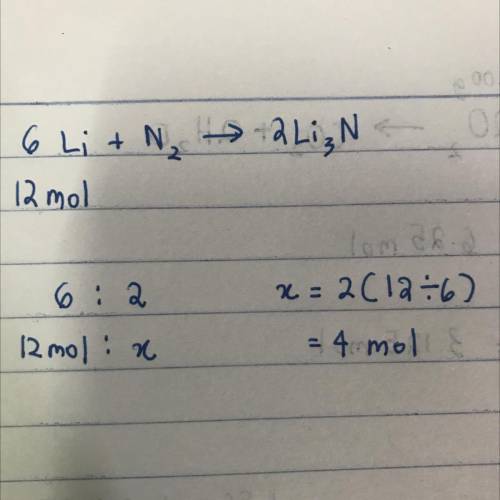Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Do you know the correct answer?
The equation below shows lithium reacting with nitrogen to produce lithium nitride.6Li + N2 Right ar...
Questions in other subjects:

Mathematics, 01.07.2019 06:30


Mathematics, 01.07.2019 06:30


Mathematics, 01.07.2019 06:30





Chemistry, 01.07.2019 06:30