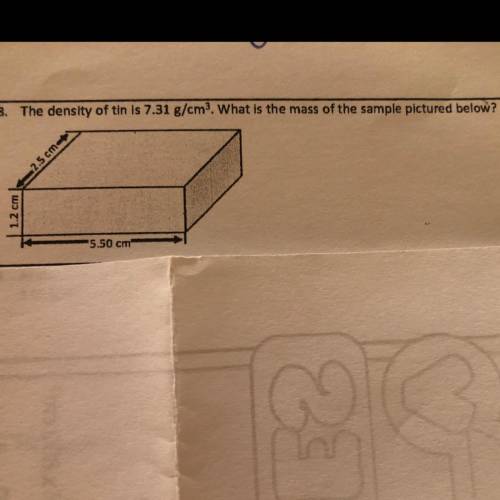
The density of tin is 7.31 g/cm? What is the mass of the sample pictured below?
2.5 cm
1.2 c...

Chemistry, 08.09.2021 09:50, izquierdohannah
The density of tin is 7.31 g/cm? What is the mass of the sample pictured below?
2.5 cm
1.2 cm
*5.50 cm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Chemistry, 25.11.2021 08:30


Social Studies, 25.11.2021 08:40

Social Studies, 25.11.2021 08:40





Social Studies, 25.11.2021 08:40






