Use the References to access important values if needed for this question.
H
A-Z
The p...

Chemistry, 06.09.2021 05:50, destiny465
Use the References to access important values if needed for this question.
H
A-Z
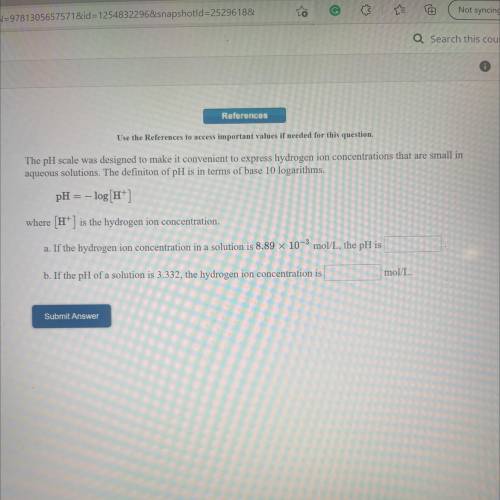
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in
aqueous solutions. The definiton of pH is in terms of base 10 logarithms.
pH = -log[H+]
where (H+) is the hydrogen ion concentration.
a. If the hydrogen ion concentration in a solution is 8.89 x 10-3 mol/L, the pH is
b. If the pH of a solution is 3.332, the hydrogen ion concentration is
mol/L.
Submit Answer

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Do you know the correct answer?
Questions in other subjects:

Computers and Technology, 25.05.2020 19:59

Mathematics, 25.05.2020 19:59

Mathematics, 25.05.2020 19:59

Mathematics, 25.05.2020 19:59



Mathematics, 25.05.2020 19:59

History, 25.05.2020 19:59