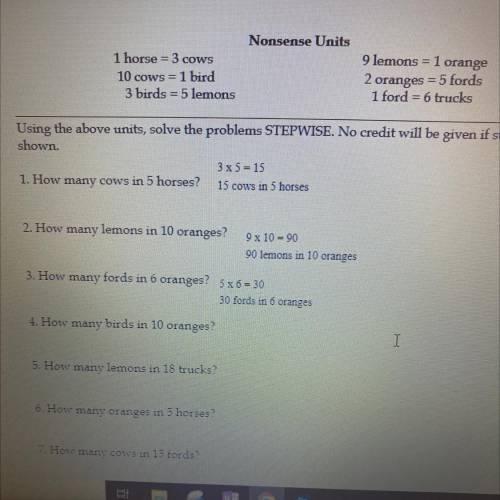
How many birds in 10 oranges? and maybe even the other questions as well!
...


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 11:00, micro7909
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow. part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer. o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part. h2o is a stronger acid than oh–, so the equilibrium lies to the right. h2o is a weaker acid than oh–, so the equilibrium lies to the left. h2o is a stronger acid than oh–, so the equilibrium lies to the left. h2o is a weaker acid than oh–, so the equilibrium lies to the right. part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer. ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-( aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part. ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right. ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left. ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right. ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left. part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer. no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part. hno2 is a stronger acid than h2o, so the equilibrium lies to the right. hno2 is a weaker acid than h2o, so the equilibrium lies to the left. hno2 is a stronger acid than h2o, so the equilibrium lies to the left. hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


SAT, 04.01.2022 19:30

Mathematics, 04.01.2022 19:30




Mathematics, 04.01.2022 19:30


Computers and Technology, 04.01.2022 19:30

History, 04.01.2022 19:30