
Chemistry, 02.09.2021 14:00, robert7248
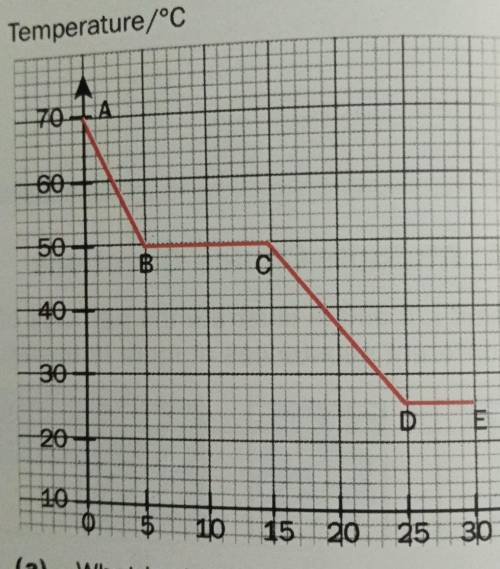
A liquid, X, was allowed to cool in air. The temperature was measured every five secds. The graph below represents the cooling curve of X.
(a) What is the melting point of X?
(b) What is the room temperature? Explain your answer.
(c) X has a boiling point of 128°C. Explain, in terms of the kinetic particle theory, what happens to the particles of X as it is heated from 100°C to 150°C.
(d) In which parts of the graph is energy being given out to the surroundings?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1


Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
Do you know the correct answer?
A liquid, X, was allowed to cool in air. The temperature was measured every five secds. The graph be...
Questions in other subjects:

Mathematics, 19.10.2019 09:50


Mathematics, 19.10.2019 09:50

Mathematics, 19.10.2019 09:50

Mathematics, 19.10.2019 09:50

Mathematics, 19.10.2019 09:50

History, 19.10.2019 09:50








