
Chemistry, 02.09.2021 07:00, minecraft37385
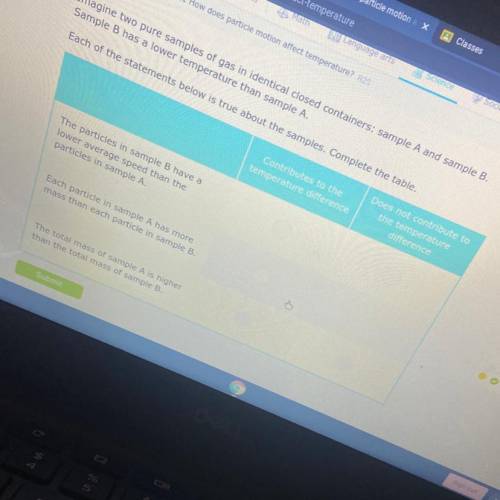
Imagine two pure samples of gas in identical closed containers: sample A and sample B.
Sample B has a lower temperature than sample A.
Each of the statements below is true about the samples. Complete the table.
Contributes to the
temperature difference
Does not contribute to
the temperature
difference
The particles in sample B have a
lower average speed than the
particles in sample A.
Each particle in sample A has more
mass than each particle in sample B.
The total mass of sample A is higher
than the total mass of sample B.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Do you know the correct answer?
Imagine two pure samples of gas in identical closed containers: sample A and sample B.
Sample B ha...
Questions in other subjects:

Biology, 30.09.2020 06:01

History, 30.09.2020 06:01

Mathematics, 30.09.2020 06:01

Mathematics, 30.09.2020 06:01

Mathematics, 30.09.2020 06:01

Chemistry, 30.09.2020 06:01

History, 30.09.2020 06:01









