Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2


Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
Do you know the correct answer?
Please Help!
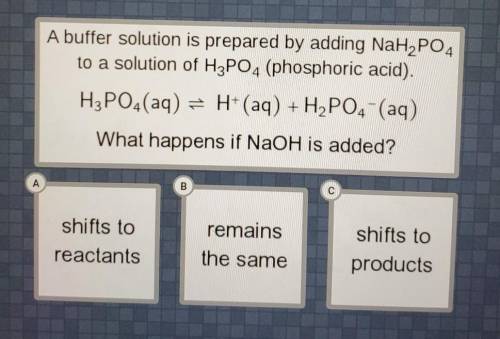
A buffer solution is prepared by adding NaH2PO4 to a solution of H3PO4 (ammonia). wha...
Questions in other subjects:


History, 17.01.2022 20:00

English, 17.01.2022 20:00

Mathematics, 17.01.2022 20:00

Health, 17.01.2022 20:00

Mathematics, 17.01.2022 20:00

Mathematics, 17.01.2022 20:00

Physics, 17.01.2022 20:00

Mathematics, 17.01.2022 20:00

Social Studies, 17.01.2022 20:00