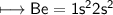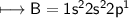Chemistry, 28.08.2021 16:30, coolfab9338
3. What is the best explanation for the decrease in first ionization energy moving from Be to B?
(A) Moving from Be to B, more electrons are added to the
atoms, thus increasing the electron-electron repulsions.
(B) Moving from Be to B, more protons in the nucleus
attract the valence electrons, making it harder to remove
the electrons.
(C) The electrons in Be are being removed from a full sub- shell, which is more stable than the half-filled sub-shell in B.
(D) The electrons in Be are located in the 2s subshell. These electrons penetrate closer to the nucleus than the 2p electrons in B.


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Do you know the correct answer?
3. What is the best explanation for the decrease in first ionization energy moving from Be to B?
(...
Questions in other subjects:






Mathematics, 03.05.2021 17:30

Mathematics, 03.05.2021 17:30

Mathematics, 03.05.2021 17:30