
Chemistry, 27.08.2021 08:10, payshencec21
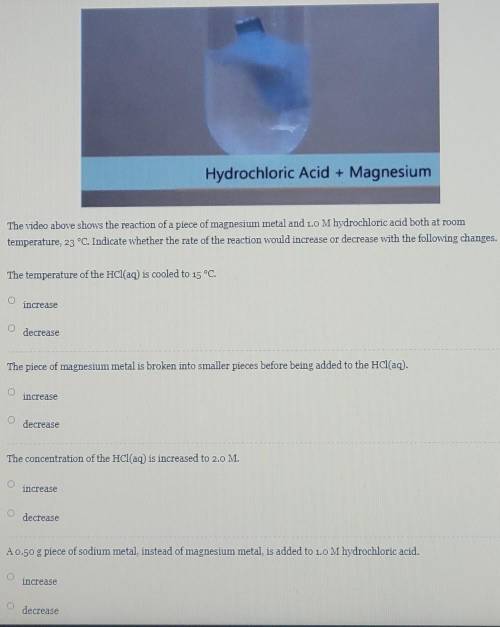
The video above shows the reaction of a piece of magnesium metal and 1.0 M hydrochloric acid both at room temperature, 23 °C. Indicate whether the rate of the reaction would increase or decrease with the following changes.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1


Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
Do you know the correct answer?
The video above shows the reaction of a piece of magnesium metal and 1.0 M hydrochloric acid both at...
Questions in other subjects:

History, 31.03.2021 20:30


Mathematics, 31.03.2021 20:30



History, 31.03.2021 20:30

History, 31.03.2021 20:30



Mathematics, 31.03.2021 20:30






