
Chemistry, 23.08.2021 20:10, TheBurntToast
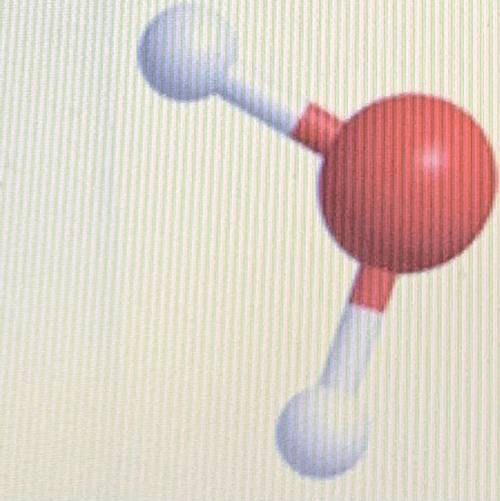
This is a model of a water molecule which has two hydrogen atoms and one oxygen atom. Why ISN'T water considered to be a MIXTURE?
A.
Because the Hydrogen & Oxygen (or O & H) atoms bonded chemically
B.
Because the Hydrogen & Oxygen atoms are easily separated

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 15:30, christopherluckey7
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
Do you know the correct answer?
This is a model of a water molecule which has two hydrogen atoms and one oxygen atom. Why ISN'T wate...
Questions in other subjects:




Mathematics, 19.09.2019 20:30



Mathematics, 19.09.2019 20:30








