to a solution of H3PO4 (phosphoric acid).

Chemistry, 15.08.2021 03:50, lolomgwtfnvm4
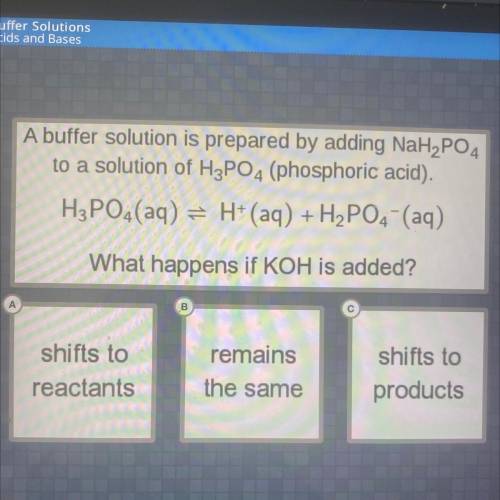
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
H3PO4 (aq) = H+ (aq) + H2PO4 (aq)
What happens if KOH is added?
remains
shifts to
reactants
shifts to
products
the same

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, cefindley14
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
to a solution of H3PO4 (phosphoric acid).
Questions in other subjects:


Biology, 17.10.2019 08:10

Social Studies, 17.10.2019 08:10

Mathematics, 17.10.2019 08:10




Mathematics, 17.10.2019 08:10


English, 17.10.2019 08:10






