3. Based on the following balanced equation:
2 C H20 + 13 02 8 CO2 + 10 H2O
a. How many moles...

Chemistry, 15.08.2021 01:00, lorraneb31
3. Based on the following balanced equation:
2 C H20 + 13 02 8 CO2 + 10 H2O
a. How many moles of O2 are required to react completely with 4.00 moles of C4H10?
3.00 6.00 8.00 10.00 13.00 16.00 20,00 23,00 26.00
b. How many moles of CO2 are produced after complete reaction of 4.00 moles of C H10?
3.00 6.00 8.00 10.00 13,00 16.00 20.00 23.00 26.00
c. How many moles of H2O are produced after complete reaction of 4.00 moles of C&H10?
3.00 6.00 8.00 10.00 13.00 1.6.00 20.00 23,00 26.00

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 06:30, morganzahn16
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
Do you know the correct answer?
Questions in other subjects:



History, 20.02.2020 18:05





Physics, 20.02.2020 18:06



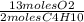 = 26 moles O2
= 26 moles O2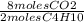 = 16 moles CO2
= 16 moles CO2 = 20 moles H20
= 20 moles H20



