
Chemistry, 12.08.2021 21:10, alupton4887
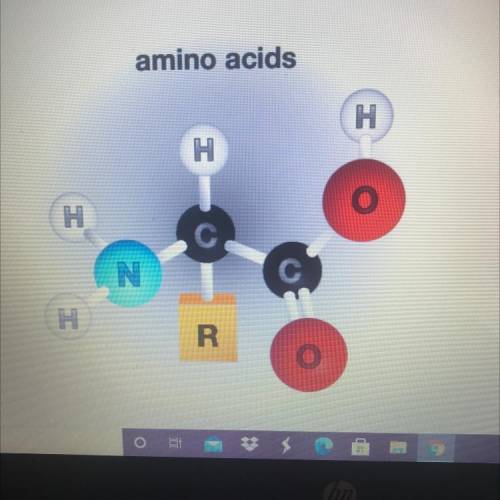
Which option explains why this amino acid can act
both an acid and a base?
(1 point)
It contains both an amino functional group and a carboxyl functional group. In a solution, the amino group can
increase acidity, while the carboxyl group can decrease acidity
It contains both an amino functional group and a methyl functional group. In a solution, the amino group can
increase acidity, while the methyl group can decrease acidity
It contains both an amino functional group and a carboxyl functional group. In a solution, the amino group can
decrease acidity, while the carboxyl group can increase acidity
It contains both an amino functional group and a methyl functional group. In a solution, the amino group can
decrease acidity, while the methyl group can increase acidity

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
Do you know the correct answer?
Which option explains why this amino acid can act
both an acid and a base?
(1 point)
It...
(1 point)
It...
Questions in other subjects:




Geography, 22.09.2019 17:30



Mathematics, 22.09.2019 17:30



Physics, 22.09.2019 17:30






