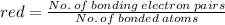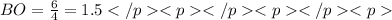Chemistry, 10.08.2021 02:10, vtrvfrfvrvfvnkjrf
The average bond order is the number of bonds between two atoms taking into account resonance.
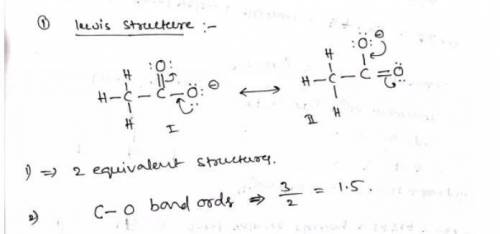
a. Draw a Lewis structure for the nitrite ion and answer the questions below.
1. there are equivalent Lewis structures for NO2-.
2. the average N-O bond order is .
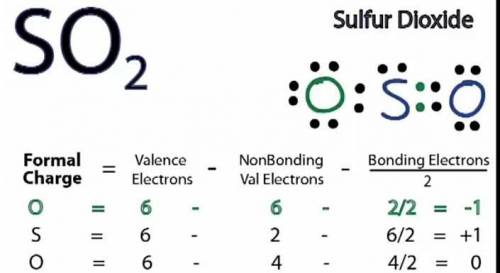
b. Draw a Lewis structure for sulfur dioxide and answer the questions below.
1. there are equivalent Lewis structures for SO2.
2. the average S-O bond order is .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
The average bond order is the number of bonds between two atoms taking into account resonance.
a....
Questions in other subjects:

Advanced Placement (AP), 05.07.2019 08:30





History, 05.07.2019 08:30

Biology, 05.07.2019 08:30

English, 05.07.2019 08:30

Mathematics, 05.07.2019 08:30