
Chemistry, 09.08.2021 14:00, ilovecatsomuchlolol
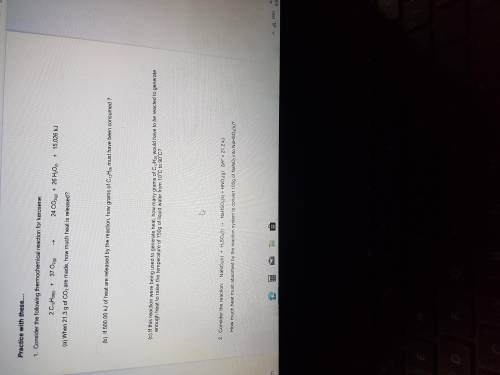
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l)+15,026kj
A. When 21.3g of CO2 are made, how much heat is released ?
B. If 500.00kj of heat are released by the reaction, how many grams of C12H26 must be produced?
C. If this reaction were to being used to generate heat , how many grams of C12H26 would have to be reacted to generate enough heat to raise the temperature of 750g of liquid water from 10 degrees Celsius to 90 degrees celsius ?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3


Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
Do you know the correct answer?
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l...
Questions in other subjects:



History, 27.01.2020 23:31


History, 27.01.2020 23:31



Geography, 27.01.2020 23:31







