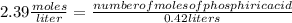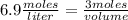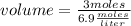Chemistry, 09.08.2021 01:20, ayoismeisjuam
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according to
the equation:
H3PO4(aq) + 3NaOH(aq) + Na3PO4(aq) + 3H2O(aq)
A) O 0.05 L
B) O6.93 L
C) O0.44 L
D) 03.01 L
E) 436.43 L


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1


Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
Do you know the correct answer?
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according...
Questions in other subjects:



Arts, 10.12.2020 21:50



History, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50



Mathematics, 10.12.2020 21:50


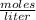 .
.