
Chemistry, 07.08.2021 01:00, radaishasmithoxngbj
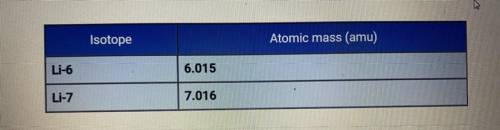
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of lithium, how do the relative abundances of the
isotopes compare?
A. Li-6 is much more abundant than Li-7.
B. They are about the same.
C. Li-7 is much more abundant than Li-6.
D. Li-7 is slightly more abundant than Li-6.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
Do you know the correct answer?
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of l...
Questions in other subjects:




Mathematics, 18.03.2021 23:40

Social Studies, 18.03.2021 23:40


English, 18.03.2021 23:40


Mathematics, 18.03.2021 23:40






