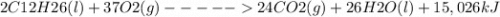Chemistry, 06.08.2021 14:00, leylaanddade
Consider the following thermochemical reaction for kerosene:
2 C12H26(l) + 37 O2(g) 24 CO2(g) + 26 H2O(l) + 15,026 kJ
(a) When 21.3 g of CO2 are made, how much heat is released?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1
Do you know the correct answer?
Consider the following thermochemical reaction for kerosene:
2 C12H26(l) + 37 O2(g) 24 CO2(g) + 2...
Questions in other subjects:



Mathematics, 08.04.2020 01:57


Mathematics, 08.04.2020 01:57



Mathematics, 08.04.2020 01:57