
Chemistry, 06.08.2021 04:40, rogersdeloris1ovgm3b
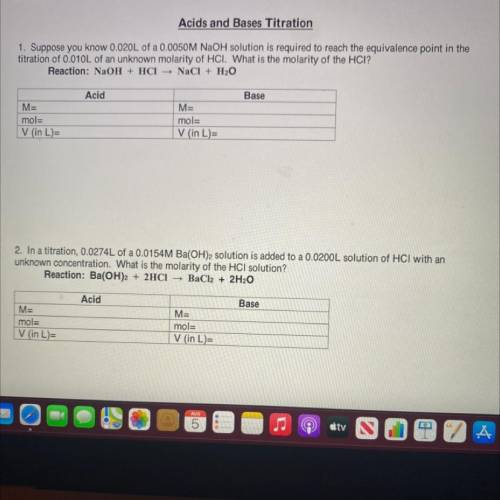
Acids and Bases Titration
1. Suppose you know 0.020L of a 0.0050M NaOH solution is required to reach the equivalence point in the
titration of 0.010L of an unknown molarity of HCI. What is the molarity of the HCI?
Reaction: NaOH + HCl NaCl + H2O
Acid
Base
M=
mol=
V (in L)=
M=
mol=
V (in L)=

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
Do you know the correct answer?
Acids and Bases Titration
1. Suppose you know 0.020L of a 0.0050M NaOH solution is required to reac...
Questions in other subjects:



Mathematics, 08.12.2021 04:30




Mathematics, 08.12.2021 04:30


Mathematics, 08.12.2021 04:30






