
It is often possible to change a hydrate into an anhydrous compound by heating it to drive off the water (dehydration). A 43.19 gram sample of a hydrate of MgBr2 was heated thoroughly in a porcelain crucible, until its weight remained constant. After heating, 27.21 grams of the anhydrous compound remained. What is the formula of the hydrate?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 09:30, carnations
What lessons does the history and study of the periodic table offer to other fields of science, and the pursuit science more generally
Answers: 3
Do you know the correct answer?
It is often possible to change a hydrate into an anhydrous compound by heating it to drive off the w...
Questions in other subjects:






Chemistry, 02.01.2020 22:31





 .
. denote the number of
denote the number of  formula units for every
formula units for every  formula unit. The formula of the hydrate would be
formula unit. The formula of the hydrate would be  .
. :
: 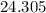 .
. :
: 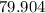 .
. :
:  .
. :
: 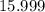 .
. .
. .
.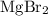 . There was
. There was 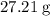 of this compound. Calculate the number of moles of formula units in that much of this compound:
of this compound. Calculate the number of moles of formula units in that much of this compound: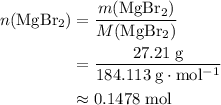 .
.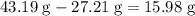 of water
of water 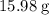 of
of 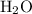 :
: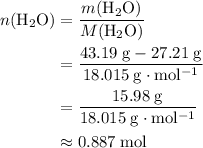 .
. of
of 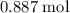 of
of  .
.




