4
D:
According to the phase diagram for H20, what happens to the phases of
water at 1 a...

4
D:
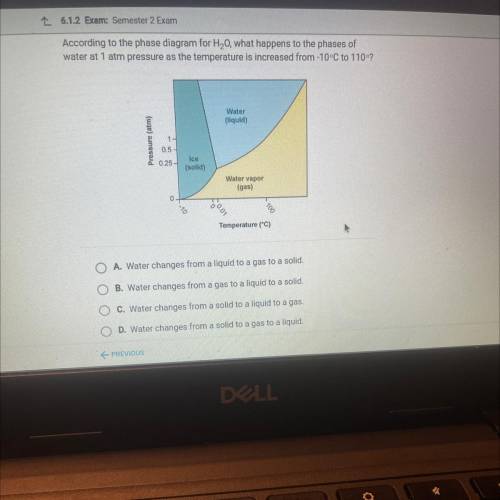
According to the phase diagram for H20, what happens to the phases of
water at 1 atm pressure as the temperature is increased from-10-C to 1102
Water
liquid
Pressure (atm)
0.5
025-
Ice
(solid)
Water vapor
(gas)
0
Temperature (°C)
O A. Water changes from a liquid to a gas to a solid
O B. Water changes from a gas to a liquid to a solid.
O C. Water changes from a solid to a liquid to a gas.
O D. Water changes from a solid to a gas to a liquid

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 02.05.2021 17:30



History, 02.05.2021 17:30


Physics, 02.05.2021 17:30




Physics, 02.05.2021 17:30






