
Chemistry, 05.08.2021 06:50, Dulcecandyy9396
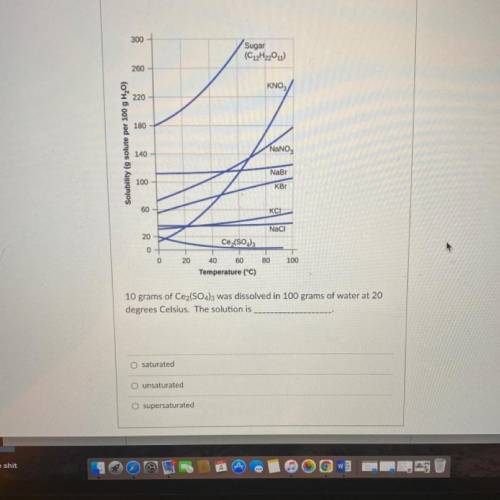
10 grams of Ce2(SO4)3 was dissolved in 100 grams of water at 20
degrees Celsius. The solution is
O saturated
O unsaturated
O supersaturated

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3


Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
Do you know the correct answer?
10 grams of Ce2(SO4)3 was dissolved in 100 grams of water at 20
degrees Celsius. The solution is
Questions in other subjects:




Mathematics, 22.09.2020 14:01






English, 22.09.2020 14:01






