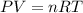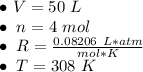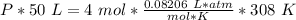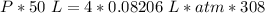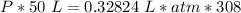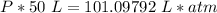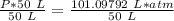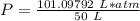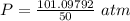Chemistry, 03.08.2021 21:10, rakanmadi87
What is the pressure of 4 moles of helium in a 50 L tank at 308 K?
Use PV = nRT.
A. 24.64 atm
B. 0.13 atm
O C. 0.51 atm
D. 2.02 atm

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:10, NavyCo
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 06:30, shateece
(04.05 hc) analyze the given diagram of the carbon cycle below. an image of carbon cycle is shown. the sun, a cloud, two trees, one on the left and the other on the right, an animal, lake, and a factory are shown in the image. an arrow is shown from the sun towards the left tree marked a. the sun is marked b. there is an arrow from the air above the clouds, marked c, towards the left tree. an arrow from a location close to the ground marked d points towards dead organisms, which is a label under the animal. an arrow marked e points from the right tree straight up to the clouds. an arrow marked f points from the animal straight up to the clouds. an arrow marked g points from the factory towards the air above the clouds, c. there is an arrow pointing from the air to the lake labeled carbonates in water, an arrow pointing down from dead organisms to fossils and fossil fuels, and an arrow from fossils to the factory. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer.
Answers: 2

Chemistry, 23.06.2019 07:00, MathChic68
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
Do you know the correct answer?
What is the pressure of 4 moles of helium in a 50 L tank at 308 K?
Use PV = nRT.
A. 24.64 atm...
A. 24.64 atm...
Questions in other subjects:

English, 01.10.2019 02:00


Social Studies, 01.10.2019 02:00




Biology, 01.10.2019 02:00



Mathematics, 01.10.2019 02:00