
Chemistry, 02.08.2021 17:10, andreamarie2004amg
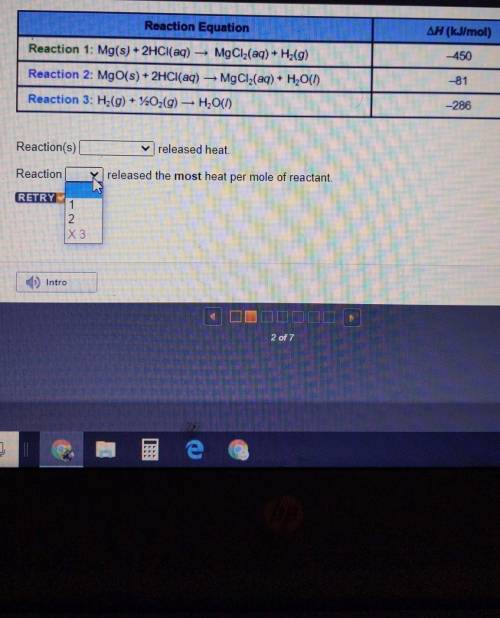
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(s) + 2HCl(aq) — MgCl2(aq) + H2O(1) Reaction 3: H2(g) + 220,(9) — H2O(1) -81 -286
Reaction(s)_released heat. W
Reaction_released the most heat per mole of reactant

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 15:30, ashtonviceoxd21i
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b. colder climates near the equator c. large waves on the cost of europe d. warm climates in northern europe
Answers: 1
Do you know the correct answer?
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(...
Questions in other subjects:


History, 19.11.2019 14:31

History, 19.11.2019 14:31




Mathematics, 19.11.2019 14:31

Mathematics, 19.11.2019 14:31


Mathematics, 19.11.2019 14:31






