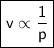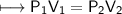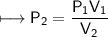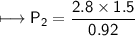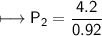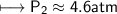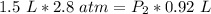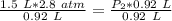Chemistry, 01.08.2021 06:00, emokid7822
At a constant temperature, a sample of gas occupies 1.5 L at a pressure of 2.8 ATM. What will be the pressure of this sample, in atmospheres, if the new volume is 0.92 L?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, ashgold324
If this equation was completed which statement would it best support
Answers: 2


Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
Do you know the correct answer?
At a constant temperature, a sample of gas occupies 1.5 L at a pressure of 2.8 ATM. What will be the...
Questions in other subjects:

English, 26.03.2020 06:58

Mathematics, 26.03.2020 06:58

Mathematics, 26.03.2020 06:58

Biology, 26.03.2020 06:59


Spanish, 26.03.2020 06:59

Mathematics, 26.03.2020 06:59

Chemistry, 26.03.2020 06:59

Arts, 26.03.2020 07:00