Chemistry, 31.07.2021 08:40, kokilavani
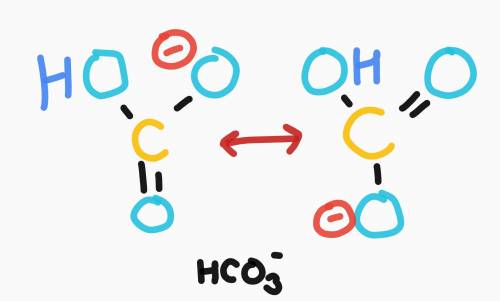
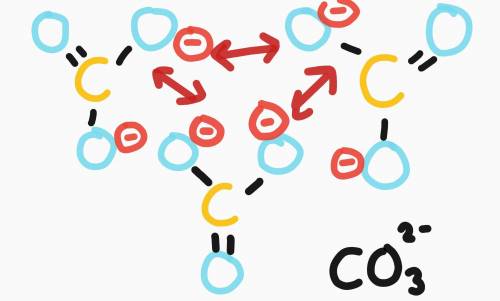
Carbonic acid (H₂CO₃) is a polyprotic acid. When carbonic acid dissolves in water, which is higher, the concentration of HCO₃- ions or the concentration of CO₃²- ions? Please explain!

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3
Do you know the correct answer?
Carbonic acid (H₂CO₃) is a polyprotic acid. When carbonic acid dissolves in water, which is higher,...
Questions in other subjects:

Biology, 05.03.2021 09:10

Mathematics, 05.03.2021 09:10

Chemistry, 05.03.2021 09:10



Computers and Technology, 05.03.2021 09:10

Mathematics, 05.03.2021 09:10

Mathematics, 05.03.2021 09:10


Mathematics, 05.03.2021 09:10