
Chemistry, 30.07.2021 14:20, ddddre3909
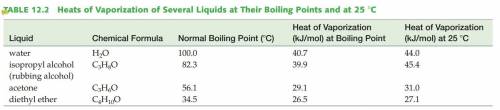
According to the Vaporization Heat table, the heat needed for 1 mol of H2O to evaporate at 100°C is 40.7KJ and 44.0KJ/mol is needed to evaporate H2O at 25°C. Thus 44.0-40.7=3.7KJ is the energy needed to heat H2O to 100°C from 25°C.
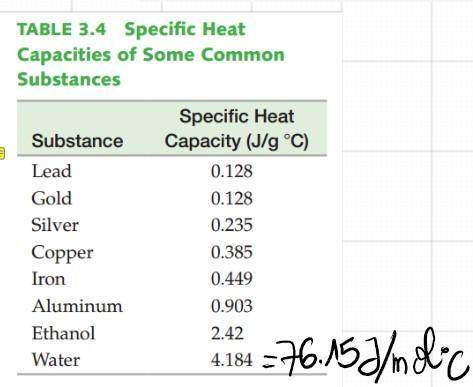
However, according to the heat capacity of H2O, 3.7KJ will only warm the water by ~+43°C, which is not enough to reach 100°C starting from 25°C!
Am I missing something?!

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, jbarbie3
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
Do you know the correct answer?
According to the Vaporization Heat table, the heat needed for 1 mol of H2O to evaporate at 100°C is...
Questions in other subjects:

Mathematics, 28.09.2021 22:00


Mathematics, 28.09.2021 22:00

History, 28.09.2021 22:00



English, 28.09.2021 22:00



Mathematics, 28.09.2021 22:00






