
Chemistry, 28.07.2021 03:30, MNBASKETBALL838
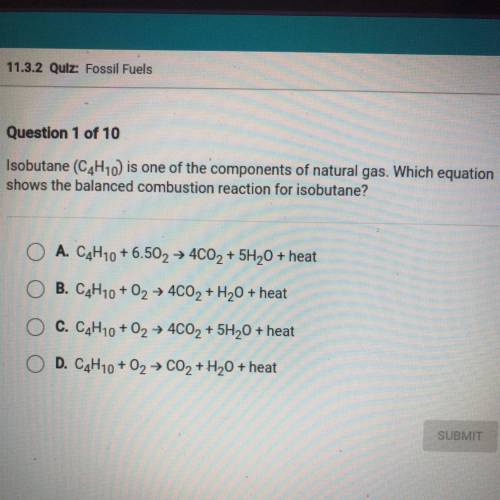
Isobutane (C4H10) is one of the components of natural gas. Which equation
shows the balanced combustion reaction for isobutane?
A. C4H10 +6.502 + 4CO2 + 5H20 + heat
B. C4H10 + O2 + 4C02 + H2O + heat
C. C4H10 + O2 + 4CO2 + 5H20 + heat
D. C4H10 + 02 → CO2 + H2O + heat

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, marknjenbennetp3j1v1
Listen base your answer to the question on the information below. propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below. c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol. what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
Do you know the correct answer?
Isobutane (C4H10) is one of the components of natural gas. Which equation
shows the balanced combus...
Questions in other subjects:

Mathematics, 21.02.2021 07:00


History, 21.02.2021 07:00

Mathematics, 21.02.2021 07:00

World Languages, 21.02.2021 07:00


Health, 21.02.2021 07:00

Mathematics, 21.02.2021 07:00

Mathematics, 21.02.2021 07:00








