
Chemistry, 27.07.2021 06:30, MayFlowers
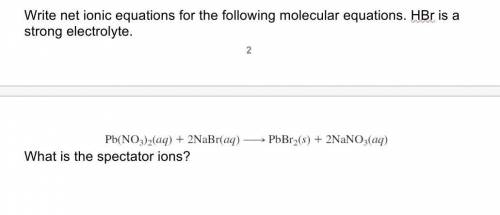
Write net ionic equations for the following molecular equations. HBr is a strong electrolyte. Pb(NO3)2(aq) + 2NaBr(aq) → PbBr2(s) + 2NaNO3(aq) What is the spectator ions? Q5 4 points How many milliliters of 0.250 M KMnO4 are needed to react with 3.36 g of iron(II) sulfate, FeSO4? The reaction is as follows: 10FeSO4(aq) + 2KMnO4(aq) + 8H2SO4(aq) 5Fe2(SO4)3(aq) + 2MnSO4(aq) + K2SO4(aq) + 8H2O(l)

Answers: 3
Other questions on the subject: Chemistry




Do you know the correct answer?
Write net ionic equations for the following molecular equations. HBr is a strong electrolyte. Pb(NO3...
Questions in other subjects:

Mathematics, 03.11.2020 21:40



Mathematics, 03.11.2020 21:40

Geography, 03.11.2020 21:40


Social Studies, 03.11.2020 21:50


Mathematics, 03.11.2020 21:50







