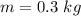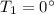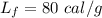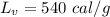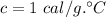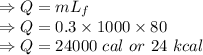Chemistry, 27.07.2021 04:40, theledfords855
Calculate the heat change in calories for melting of 0.30 kg of water at 0*C. The
heat of fusion for water is 80 cal/g. The heat of vaporization of water is 540 cal/g.
The specific heat capacity of water is 1.00 cal/g*C.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:50, ichabella2010
Which phrase best describes the rock's texture? 1.jagged grains 2.coarse grains 3.rounded grains 4.non-banded grains
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
Do you know the correct answer?
Calculate the heat change in calories for melting of 0.30 kg of water at 0*C. The
heat of fusion fo...
Questions in other subjects:



Computers and Technology, 24.09.2019 00:00







Social Studies, 24.09.2019 00:00