What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded a...

Chemistry, 26.07.2021 22:20, joselinegarciaowyrpf
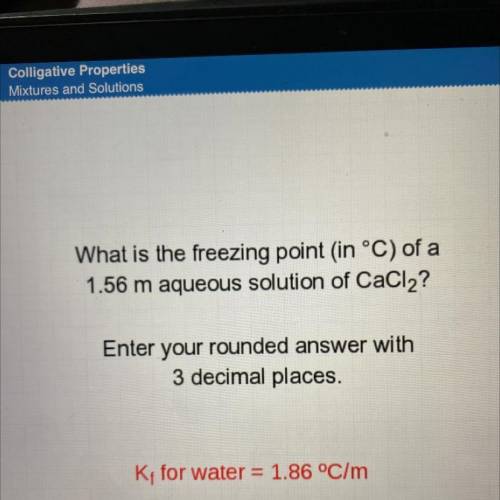
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
K; for water = 1.86 °C/m

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 07.06.2020 13:40

Mathematics, 07.06.2020 13:40

World Languages, 07.06.2020 13:40

Mathematics, 07.06.2020 13:40

World Languages, 07.06.2020 13:40


History, 07.06.2020 13:40

Mathematics, 07.06.2020 13:40

Mathematics, 07.06.2020 13:40






