
Chemistry, 25.07.2021 06:20, brooklynunderwood46
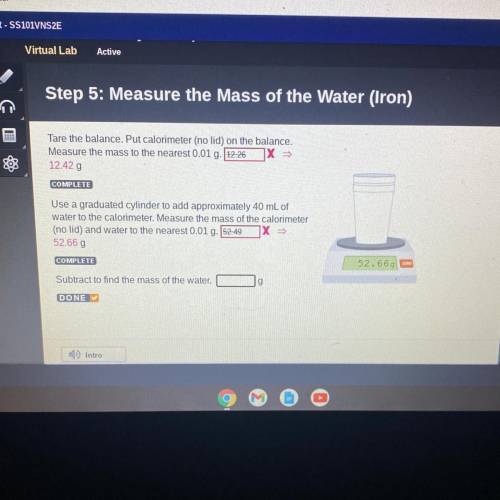
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g. 12.26 X
12.42 g
COMPLETE
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the calorimeter
(no lid) and water to the nearest 0.01 g. 52.49
52.66 g
COMPLETE
52.669
Subtract to find the mass of the water.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Do you know the correct answer?
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the bala...
Questions in other subjects:

History, 11.11.2019 22:31



Mathematics, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31

Biology, 11.11.2019 22:31

Health, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31


Mathematics, 11.11.2019 22:31






