
Chemistry, 24.07.2021 06:40, steven2669
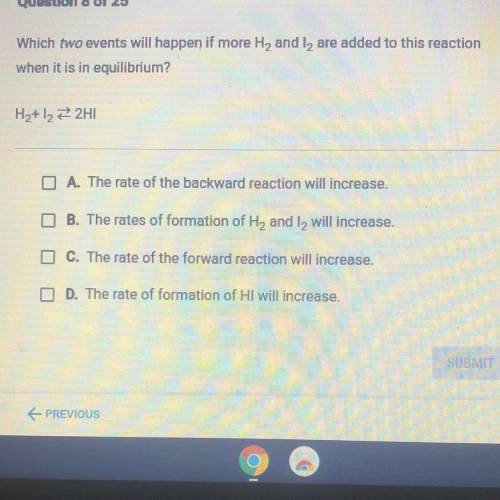
Which we events will happen if more H, and t, are added to this reaction
when it is in equilibrium?
A. The rate of the backward reaction will increase
The rates of formation of He and I will increase
0. The rate of the forward reaction will increase
D. The rate of formation of HI will increase

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, britotellerialuis
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
Do you know the correct answer?
Which we events will happen if more H, and t, are added to this reaction
when it is in equilibrium?...
Questions in other subjects:

Mathematics, 18.05.2021 19:20


Mathematics, 18.05.2021 19:20












