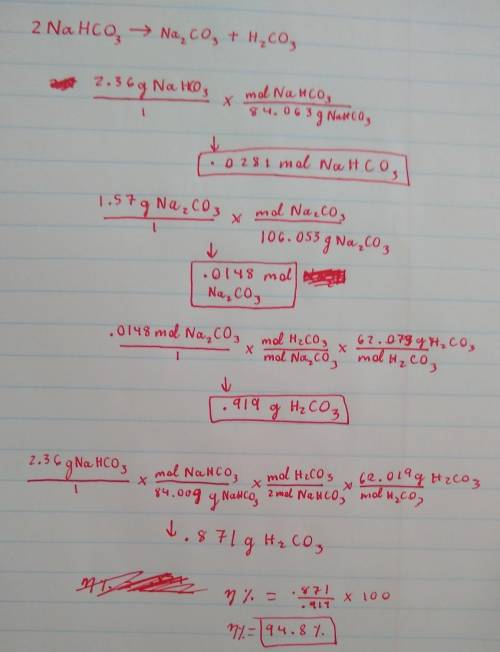ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na...

Chemistry, 24.07.2021 01:00, crookdamian21
ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na2CO3 + H2CO3
In this experiment, carbon dioxide and water vapors combine to form H2CO3. After decomposition, the Na2CO3 had a mass of 1.57 grams.
Determine the mass of the H2CO3 produced.
Calculate the percentage yield of H2CO3 for the reaction. Show your work or describe the calculation process in detail.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 03.02.2021 19:30

Mathematics, 03.02.2021 19:30

Mathematics, 03.02.2021 19:30


Mathematics, 03.02.2021 19:30


Mathematics, 03.02.2021 19:30