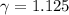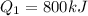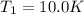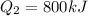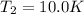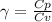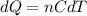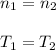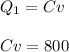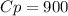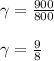A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant volume. The same quantity of gas requires 900 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant pressure. What is the adiabatic gas constant of this gas

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1


Chemistry, 23.06.2019 17:30, noglapotato
If 2.40 moles of gas are held at a temperature of 97.0 °c in a container with a volume of 45.0 l, what is the pressure of the gas?
Answers: 1

Chemistry, 24.06.2019 01:00, ste78
What is an effect of gravity on objects on the surface of earth? check all that apply. objects “fall” toward the center of earth. objects are pulled by earth but do not pull on earth. objects accelerate at a rate of 9.8 m/s each second. objects travel at a rate of 9.8 m/s toward the center of earth. objects are pulled by earth more strongly than they pull on earth.
Answers: 1
Do you know the correct answer?
A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas i...
Questions in other subjects:

Mathematics, 04.02.2020 05:44

Chemistry, 04.02.2020 05:44

Mathematics, 04.02.2020 05:44


Mathematics, 04.02.2020 05:44



Mathematics, 04.02.2020 05:44

Mathematics, 04.02.2020 05:44