
Chemistry, 21.07.2021 01:00, QueenZenobia
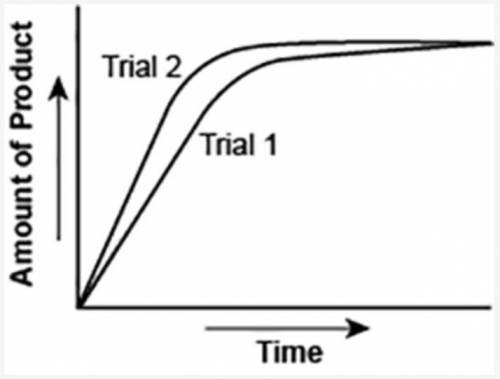
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A: Trial 1, because the average rate of the reaction is lower.
B: Trial 1, because this reaction lasted for a longer duration than Trial 2.
C: Trial 2, because this reaction was initially fast and later slowed down.
D: Trial 2, because the volume of product formed per unit time was higher.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3



Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
Do you know the correct answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions in other subjects:

History, 01.09.2019 05:30





Health, 01.09.2019 05:30

English, 01.09.2019 05:30

Business, 01.09.2019 05:30

History, 01.09.2019 05:30

Social Studies, 01.09.2019 05:30






