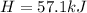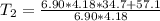Chemistry, 20.07.2021 04:10, hayden5928
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6.90kg of water at 34.7 degrees C . During the reaction 57.1kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J. g^(-1).K^(-1) . Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Do you know the correct answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6....
Questions in other subjects:



History, 27.07.2019 22:30

Mathematics, 27.07.2019 22:30






History, 27.07.2019 22:30