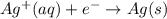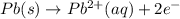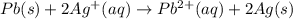Chemistry, 19.07.2021 23:20, netflixacc0107
A voltaic cell is constructed with an Ag/Ag half-cell and a Pb/Pb2 half-cell. The silver electrode is positive. Write the balanced half-reactions and the overall reaction. Include the phases of each reactant and product.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, eddsworldfrantic
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2



Chemistry, 23.06.2019 13:30, querline87
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
Do you know the correct answer?
A voltaic cell is constructed with an Ag/Ag half-cell and a Pb/Pb2 half-cell. The silver electrode i...
Questions in other subjects:

Mathematics, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00



Chemistry, 10.12.2020 01:00

Business, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00