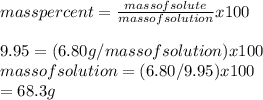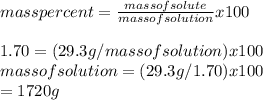Chemistry, 19.07.2021 17:30, whocares1234
Calculate the amount of water (in grams) that must be added to (a) 6.80 g of urea [(NH2)2CO] in the preparation of a 9.95 percent by mass solution: g (b) 29.3 g of MgBr2 in the preparation of a 1.70 percent mass solution: g

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
Do you know the correct answer?
Calculate the amount of water (in grams) that must be added to (a) 6.80 g of urea [(NH2)2CO] in the...
Questions in other subjects:

Mathematics, 09.03.2021 14:00



Mathematics, 09.03.2021 14:00


English, 09.03.2021 14:00


Chemistry, 09.03.2021 14:00

Advanced Placement (AP), 09.03.2021 14:00