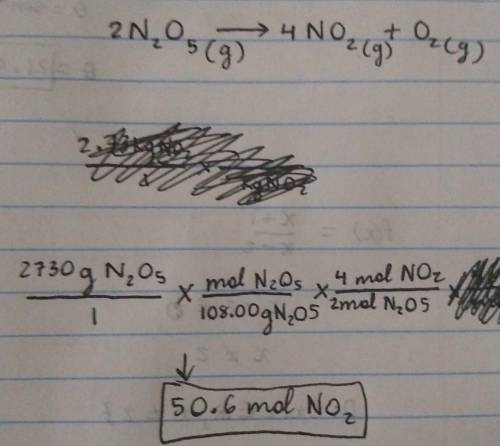Chemistry, 19.07.2021 03:40, anonymous1813
Calculate how many moles of NO2
form when each quantity of reactant completely reacts via the following reaction:
2N2O5(g)→4NO2(g)+O2(g)
2.73 kg N2O5
Express your answer using three significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
Do you know the correct answer?
Calculate how many moles of NO2
form when each quantity of reactant completely reacts via the follo...
Questions in other subjects:



Mathematics, 15.12.2020 14:00



Biology, 15.12.2020 14:00

Mathematics, 15.12.2020 14:00


English, 15.12.2020 14:00