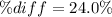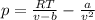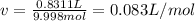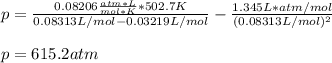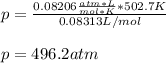Chemistry, 18.07.2021 03:50, andrea1704
According to the ideal gas law, a 9.998 mol sample of argon gas in a 0.8311 L container at 502.7 K should exert a pressure of 496.2
atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For Ar
gas, a = 1.345 L’atm/mol? and b = 3.219x10-2 L/mol.
Pideal – Puan der Waals |
Percent difference
x 100

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1


Chemistry, 23.06.2019 04:00, Mitchmorgan3816
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 10:30, 7thaohstudent
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2
Do you know the correct answer?
According to the ideal gas law, a 9.998 mol sample of argon gas in a 0.8311 L container at 502.7 K s...
Questions in other subjects:

Mathematics, 17.05.2021 16:40


History, 17.05.2021 16:40



Mathematics, 17.05.2021 16:40