
Chemistry, 17.07.2021 01:00, heyitstierney5610
Use the problem below to answer the question:
34 grams of carbon reacted with an unlimited amount of H2O. The reaction is:
C + H2O → CO + H2
The atomic mass of C is 12.01 g/mole. The atomic mass of H2 is 2.016 g/mole. Finish the problem by choosing the correct format for dimensional analysis.
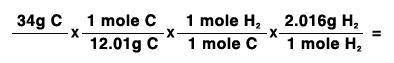
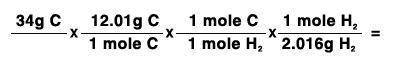
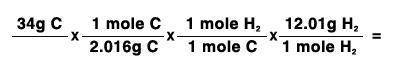

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1


Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
Use the problem below to answer the question:
34 grams of carbon reacted with an unlimited amount o...
Questions in other subjects:

English, 06.06.2021 04:50

Mathematics, 06.06.2021 04:50

Mathematics, 06.06.2021 04:50





Mathematics, 06.06.2021 04:50







