
Chemistry, 16.07.2021 23:30, Mayjay2827
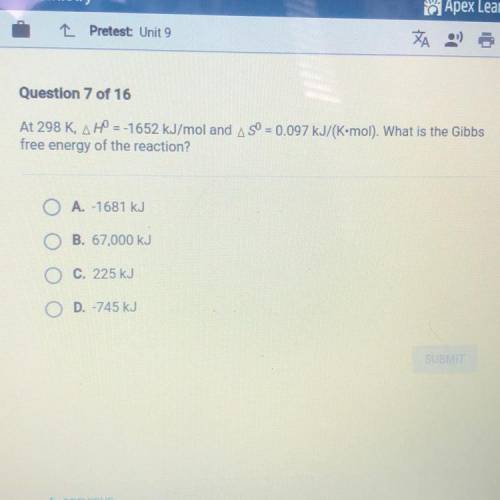
At 298 K, H = -1652 kJ/mol and S = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?
A.-1681 kJ
B. 67,000 kJ
C. 225 kJ
D. -745 kJ

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Do you know the correct answer?
At 298 K, H = -1652 kJ/mol and S = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?...
Questions in other subjects:

Mathematics, 17.09.2020 06:01

History, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01






